B 9 Bohr Model
The force responsible for the electron’s circular motion is the electric force of attraction between the electron and the proton.

B 9 bohr model. 9.27 10 joule/tesla24 m B =. Where n(E) is the electron number density, or the number of electrons per unit volume;. A) The energy emitted from a relaxing electron can have any wavelength.
11 - Xenon (Z=54) was the first noble gas. D) the nucleus. Scattering matrix elements are calculated using the channel packet method, and non-adiabatic wavepacket dynamics are determined using the split-operator method together with a unitary.
Moseley Experiment - Atomic Number;. Which statement below does NOT follow the Bohr Model?. Discovery of Fundamental Particles;.
(frequency = 3.0 x 10 9 Hz). C) at random speeds in fixed orbits. This quiz is incomplete!.
Https://youtu.be/S1LDJUu4nko “Bohr's Model of an Atom - CBSE 9.” YouTube, 30 July 18, youtu.be/S1LDJUu4nko. Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. A)2 b)8 c)18 d)10 Question 11.In an atom, the constituent electrons:.
257 follows t e Bohr model and the radius of 100Fm 257 is n times the Bohr radius, then find n. And F is the Fermi factor.The Fermi factor is the probability that the state will be filled. How to draw a Lewis Structure;.
(4) Electronic configuration of C(8)=2, 6 So, its valency is 2. According to Bohr's model of the atom, electrons behave like a. Bohr’s atomic model A.
A) in fast, random patterns. Atomic model Neils Bohr 10/7/15 - 11/18/1962 By applying Planck's Quantum theory to the Rutherford model Bohr was able to create the Bohr model of the atom. Explains the emission spectra of hydrogen atoms.
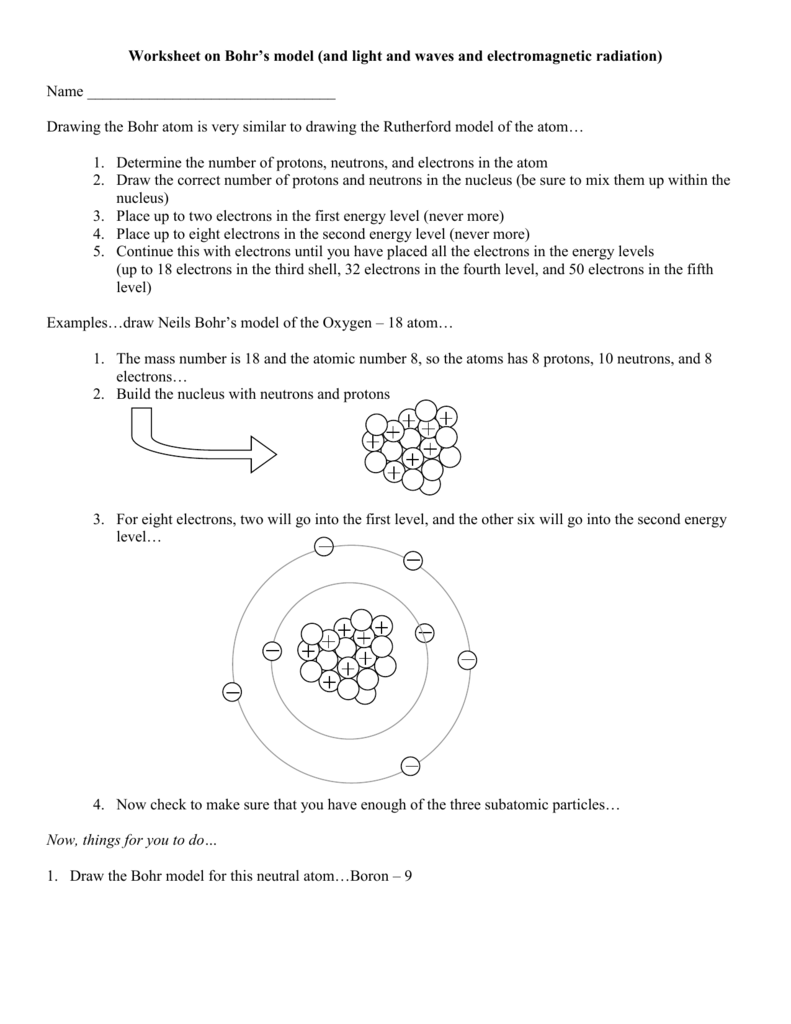
Predicts the energy levels of multi-electron atoms. Mass number of B = 9 + 8= 17 (2) The atomic number of B = Number of protons = 9 (3) Elements C and D represent a pair of isotopes because their atomic numbers are the same, but mass numbers are different. What is the magnitude of the electric force between the electron and the proton?.
The early th century brought a succession of scientific models, or theories, to describe the atom and its components. Model of a hydrogen atom. (a) The Bohr orbit with the smallest radius is called the first Bohr.
DE is the size of the energy interval;. B) in slow, random patterns. 11 - Carbon does not form a stable monatomic ion.
Bohr’s Model of Atom;. While we work to ensure that product information is correct, on occasion manufacturers may alter their ingredient lists.Actual product packaging and materials may contain more and/or different information than that shown on our Web site. Meanwhile, the study of atomic spectra—the light given off by atoms at definite wavelengths—led to the Bohr model of the atom, where electrons exist at distinct energy levels and move between these levels by absorbing and emitting discrete quanta of energy.
Rutherford Atomic Model and Bohr Atomic Model | Lecture 31. For each, describe their experiment and model of the atom. Rutherford's model of an Atom was undoubtedly a breakthrough in Atomic studies.
The quantum-mechanical model of the atom A. In the Bohr model, the electron is imagined to move in circular orbits about a stationary proton. For example, if g(E)dE is 100 available states, but F is only 5 % 5 %, then the number of.
Bohr's model shows that electrons move in paths, which are called orbits. Outline the contributions of Dalton, Thomson, Rutherford and Bohr to the present understanding of atomic structure. A Bohr Diagram is the model of an atom with the Nucleus at the center, and the.
The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. To play this quiz, please finish editing it. This presentation covers atomic structure, protons, electrons, neutrons, periodic table element tiles, and Bohr models.
Transitions in a magnetic field. Neils Bohr, the scientist that created the Bohr model, had demonstrated that electrons in atoms are in an orbit containing energy around the nucleus. These orbits differed in their distance from the nucleus and in their energy levels.
Bohr’s atomic model was different than Rutherford’s atomic model because it represented the orderly movement of _____. Look at the Bohr model of the helium atom shown below. QUESTION 6 In the Bohr model, the electron moves in a circular orbit around the nucleus with a radius of 5.29 x 10-11m.
11 - What do you suppose are the electron configuration. He also believed that the electrons closest to the nucleus have the lowest amount of energy. Students can solve NCERT Class 12 Physics Moving Charges and Magnetism MCQs Pdf with Answers to know their preparation level.
Calculate the wavelength (in nm) of a the red light emitted by a neon sign with a frequency of 4. The Bohr model, on the other hand, showed that most of the matter in an atom are concentrated in a very tiny and dense nucleus and electrons orbit the nucleus at a great distance. #43 #atom #bohr #element #tc #technetium Explore the world’s largest, free 3D model library, but first, we need some credentials to optimize your content experience.
Here the other thing changing with n is the deBroglie wavelength of the electrons, because the electron energy and momentum also change with n. In the Bohr model of the Hydrogen atom, the electron orbits the proton in a circular orbit of radius 0.529\times 10^{-10}. The model we use today is different, it puts electrons in the relative locations instead of the specific orbits.
11 - Consider the block of elements in Periods 2 to 6. D) When energy is absorbed by atoms, the electrons are promoted to higher-energy orbits. 633 nm, 158 nm, 142 nm, 704 nm, 466 nm.
B) Electrons exist in specific, quantized orbits. The gravitational interaction between the proton and the electron can be ignored. The Bohr model and the quantum mechanical model.
The shell configuration is:. The product of the synthesis reaction between sodium and chlorine gas is a. As experiments revealed more about subatomic particles, atomic models evolved from Thomson’s “plum pudding model,” to Rutherford’s nuclear model, then to Niels Bohr’s planetary model, and eventually to the currently-accepted quantum-mechanical model.
2,8,18,14,1 I made this as part of a chemistry assignment. The structure similar to that of a solar system. Give reasons for the following:.
Proposes that electrons occupy specific energy levels. Bohr’s model for Hydrogen Bohr’s model for hydrogen atom is based on the following postulates:. We recommend that you do not solely rely on the information presented and that you always read labels, warnings, and directions before using or.
Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6. Models of the Atom – – – – – + –. 11 - Although the quantum model of the atom makes.
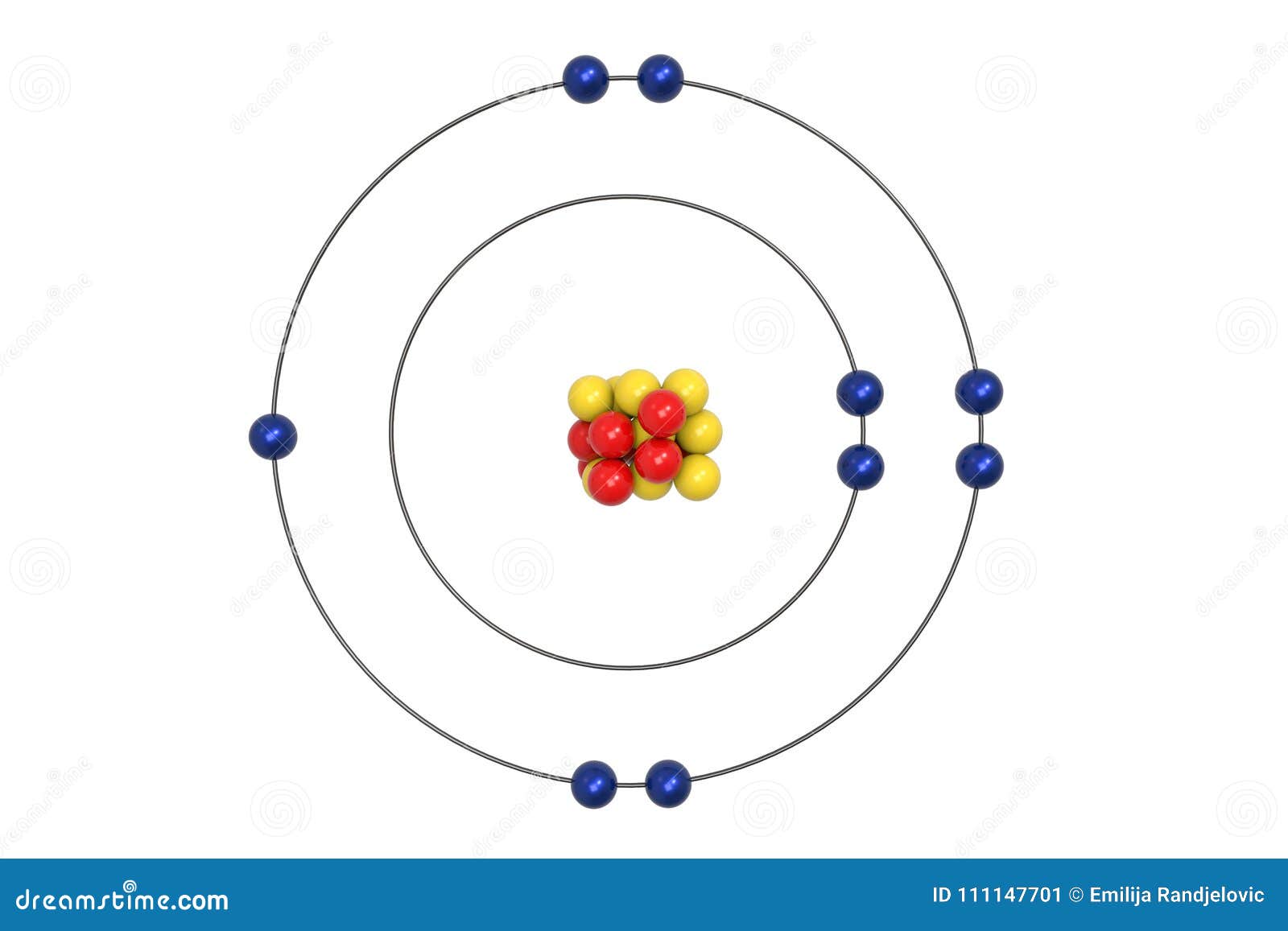
The Bohr model assumes that the electrons move in circular orbits that have quantized energies, angular momentum, and radii that are specified by a single quantum number, n = 1, 2, 3, …, but this quantization is an ad hoc assumption made by Bohr to incorporate quantization into an essentially classical mechanics description of the atom. These orbits are arranged concentrically around the nucleus. The Bohr model of carbon has a central nucleus containing six protons and six neutrons, encircled by an inner orbit of two electrons and an outer orbit of four electrons.
In 1913, the Danish scientist Niels Bohr (15–1962) refined the existing atomic model to account for the locations of the electrons. Now, what it means that the energy in an atom is quantized is. From the Bohr model we know that r n=n2a o.
( a ) 100 ( b ) 0 ( c ) 4 ( d ) 1 / 4 IIT 03 28 ) A nucleus with ma s number 2 initially at rest emits an α-particle. These paths are called orbits, stationary states or allowed energy states. Bohr suggested that electrons move _____ around the nucleus.
Chemistry 9th | Chapter 2 | Difference :. One model of a hydrogen atom, which has one proton and one electron, suggests that the proton is at rest, and the electron orbits the proton in a circular orbit of radius r = 5.29 x 10-11 m. If Rutherford’s random electron cloud model of the atom was correct, Bohr would have seen a continuous.
How many Electrons are found in any Elements Valence Shell ;. Bohr had explained that these shells were in differing energies. We use the Baranger model to compute collisional broadening and shift rates for the D 1 and D 2 spectral lines of M + Ng, where M = K, Rb, Cs and Ng = He, Ne, Ar.
B) 9 x 10 17 m c) 10 m d) 0.1 m. However, it wasn't completely correct. Sommerfeld’s Extension of Bohr Theory;.
The measurement of atomic spectra has applications in astrophysics as well as. Chapter 7 the quantum-mechanical model of the atom:. What is the energy in Joules of a mole of photons of microwave light having a frequency typical of microwave?.
The atom described by the Bohr model (note that the nucleus and electrons are not to scale). If tt is the orbit number of the electron in a hydrogen atom, the. Unit 1 pretest (p. HERE, p.123 HERE) *nuclear decay KEY *How Atoms Differ?.
It has since been superseded but remains relevant as a way to open up quantum mechanics. What part of the atom is represented by the letter Z?. A)Outermost orbit b)Next to outermost orbit c)First orbit d)Any one of its orbit Question 10.The maximum number of electrons that can be accommodated in third shell ( n = 3) is:.
Red, green, blue, purple) Next Class:. Bohr Model of the Hydrogen Atom (you will need color pencils/pens for this:. (b) uses Einstein’s photoelectric equation.
According to the Bohr model, the electrons were restricted to certain specific orbits around the nucleus of the atom. Planets orbiting the sun. It needed slight modifications.
18 Questions Show answers. The Boeing B-9 bomber was the earliest plane based on the advanced, extremely aerodynamic Monomail design of 1930 that made traditional biplane construction obsolete. First, there are two models in the atomic structure, which would be:.
11 - One of the successes of the Bohr model of the atom. According to Bohr's theory, an electron's path. G(E) is the density of states, or the number of allowed quantum states per unit energy;.
The Bohr model and all of its successors describe the properties of. 3.7x10-8 Ob.8.2x 10N 0.6.3x10-8 O d.7.4*10-8 e.2.9*10-8 QUESTION 7 Continuing, what is the kinetic energy of the electron?. 17 Electric charge Danish physicist Niels Bohr introduced a simple model for the hydrogen atom.
Concept of Shells and Sub-shells;. Transitions occur in an atom between l=2 and l=1 states in a magnetic field of 0.6T, obeying the selection rules ∆m l =0,±1. Dual Nature of Matter and Light;.
Introduce atoms and atomic structure with this PowerPoint presentation. In Bohr model of hydrogen atom, the ratio of periods of revolution of an electron in n =2 and n = 1 orbits is (a) 2 :. (c) predicts continuous emission spectra for at-oms.
1 (b) 4 :. (A Joule is enough energy to raise the temperature of 1 gram of water about a quarter of a degree Celsius.) a) 1.2 x 10 23 J b) 1.98 x 10-24 J c) 1.2 J d) 1.98 x 10-34 J. Chemical Symbols and Atomic Mass Quiz Overview of Chemical Symbol Quiz Due 10/8 & 10/9:.
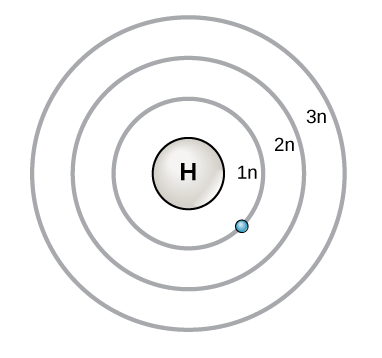
Both a and b 5. This is a bohr model of the element Technetium, Tc. As you all know, the Hydrogen atom consists of one electron and one proton.
If the Q value of. (a) The nucleus of an atom is heavy. If the wavelength before the.
Copper (II) chloride c. Light energy in a vacuum. Every electron has a permanent magnetic moment of amount μ B = 9.27 × 10 −24 J T −1 (Bohr's magneton, BM), which is parallel to its spin s.For an electron system with total angular momentum quantum number J, the magnetic moment is μ = gJ(J + ,1) 1/2 BM where g is the Landé splitting factor, and g = 1 + {J(J + 1)+ S(S + 1) − L(L + 1)/2J(J + 1)}.
Physics MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. This resource includes a 19 slide PowerPoint presentation and 2 versions of the student notes pages - full. C) When an atom emits light, electrons fall from a higher orbit into a lower orbit.
In 1913 he revolutionized the atomic structure with the "Bohr's Model" depicting the atomic structure of an atom based on the number of electrons within the atom and its properties. Energy Level Orbitals Maximum Capacity 1 1s 2 electrons 2 2s, 2p 8 electrons 3 3s, 3p, 3d 18 electrons 4 4s, 4p, 4d, 4f 32 electrons 5 Notes. B (9) The Bohr model is in sharp disagreement with the full quantum solution.
Sodium has an atomic number of 11 and an average atomic mass of 22.99 amu. It had a top speed of 186 mph (299 kph) and could outrun the fighters of the day by 5 mph (8 kph). Describes an electron probability distribution that determines the most likely.
The Bohr model of atoms (a) assumes that the angular momentum of elec-trons is quantized. A)Bohr b)Chadwick c)Rutherford d)Dalton Question 9.In an atom valence electron are present in:. (d) predicts the same emission spectra for all types of atoms.
With the positively charged nucleus in the center.
2

What Is The Purpose Of Drawing A Bohr Model Of An Atom Brainly Com
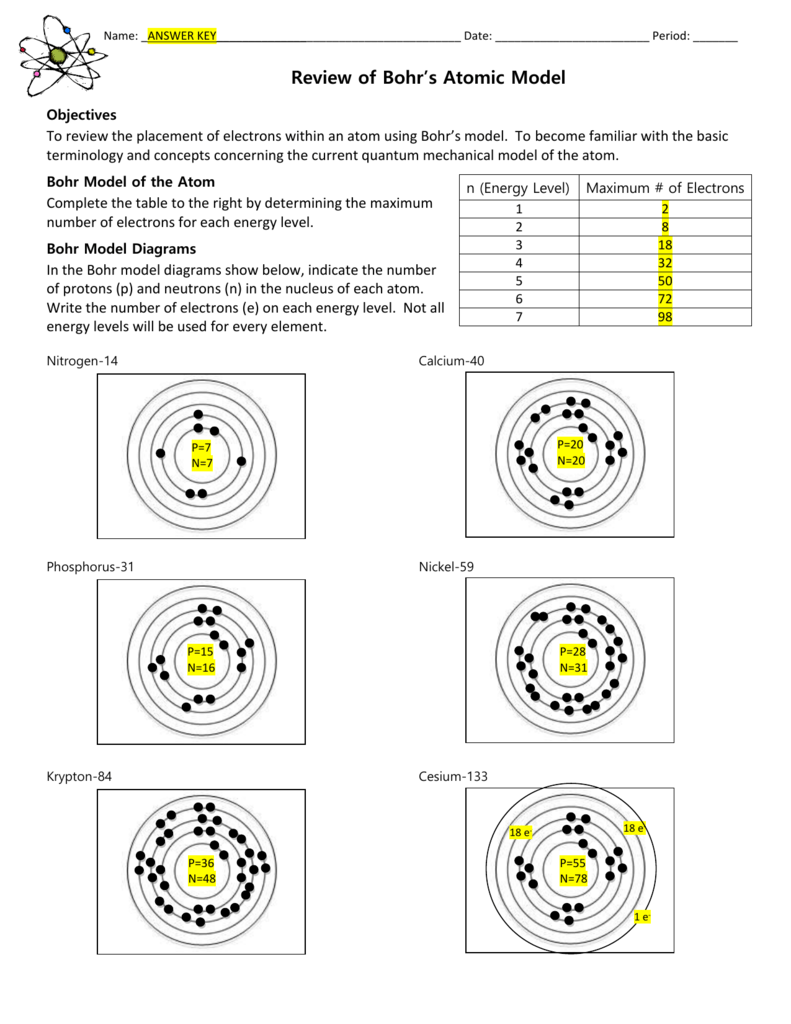
Review Of Bohr Models Answer Key
B 9 Bohr Model のギャラリー

Atomic Structure

Niels Bohr Wikipedia

Bohr Model Energy Levels Video Khan Academy

For The Energy Levels In An Atom Which One Of The Following Statements Is Correct

High School Chemistry The Bohr Model Wikibooks Open Books For An Open World

Chapter 9 Electrons And The Periodic Table Moorpark College
Bohr Model Wikipedia
2
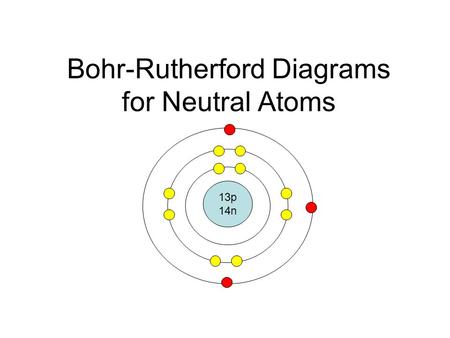
Bohr Models Ppt Download

High School Chemistry The Bohr Model Wikibooks Open Books For An Open World
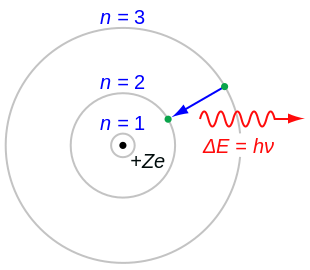
Bohr Model Wikipedia

Bohr S Atomic Model And Its Limitations Study Bohr S Theory On Byju S

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit
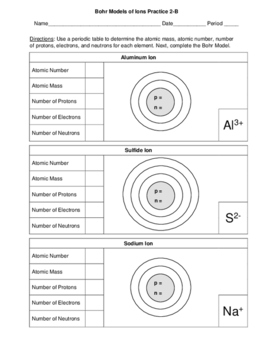
Bohr Models Of Ions 2 Worksheets 3 Skill Level Versions Of Each 12 Pages
Worksheet On Bohr S Model And Light And Waves And
Fluorine Atom Bohr Model With Proton Neutron And Electron Stock Illustration Illustration Of Elements Core

Solved 8 In The Bohr Model Of The Atom A Electrons Tra Chegg Com
Q Tbn 3aand9gcsmaz 1eyztdqbvapeouj0fznnxyysqsglrb8j4ou2j8icjvqgp Usqp Cau

Atomic Structure Bohr Model Worksheet Kids Activities
Q Tbn 3aand9gcq6wim Zxxp Omtr8zi8snmb Cd0huawzf Kx6b7ws9fd P0dv Usqp Cau
Atoms Molecules Lab The Biology Primer

Bohr S Molecular Model A Century Later Physics Today Vol 67 No 1

Amazon Com Niels Bohr Atom Model Hanging Mobile 9 Inches Steel Handmade In Denmark By Flensted Home Kitchen

Solved 9 Use The Bohr Model Generalized For Gravity For Chegg Com
Bohr Model Diagrams Of Atoms Ppt Download

Review Grade 9 Chemistry
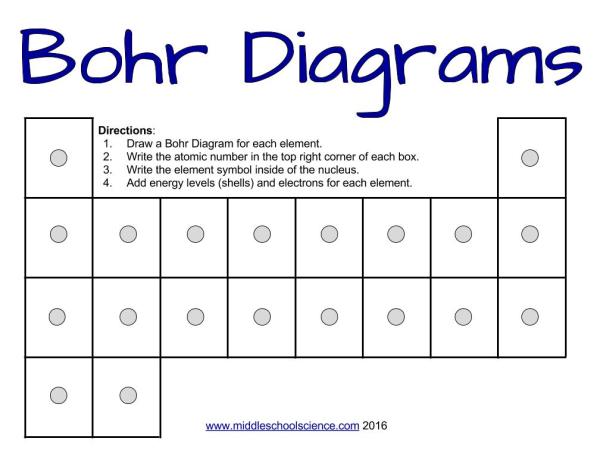
How To Draw Bohr Diagrams A Step By Step Tutorial Middle School Science Blog

Answer Which Energy Gap In The Bohr Model Clutch Prep
Bohr Model Of The Atom Overview And Examples

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science
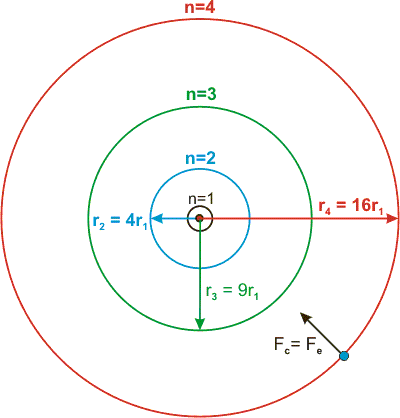
Chapter 2 Quantum Theory
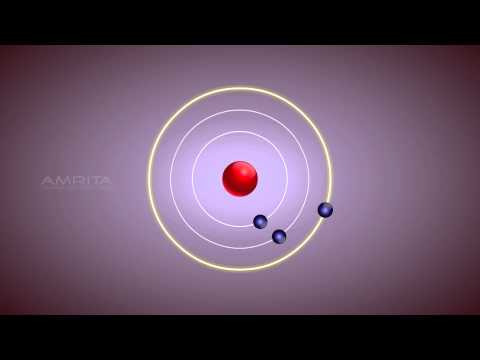
Bohr S Model Of An Atom Class 9 Tutorial Youtube
2
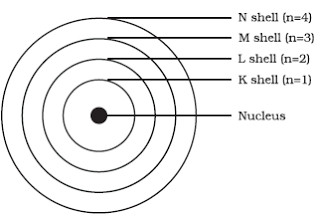
Bohr S Model Of An Atom Class 9 Structure Of An Atom

The Bohr Model And Atomic Spectra Video Lesson Transcript Study Com
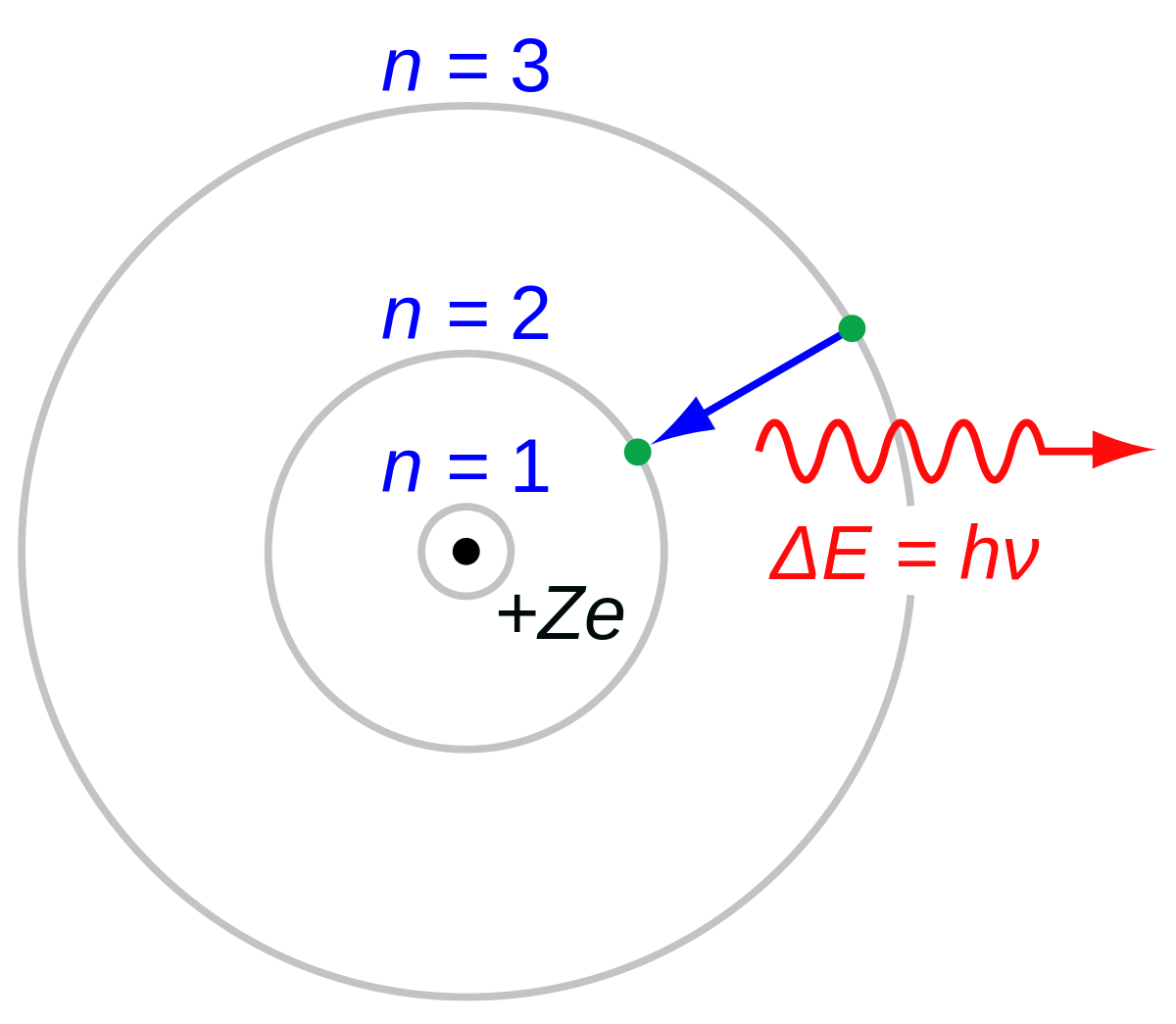
Bohr Model Wikipedia

For A Hydrogen Atom In Its Ground State Use The Bohr Model To Compute A The Orbital Speed Youtube

A Using The Bohr S Model Calculate The Speed Of The Electron In A Hydrogen Atom In The N 1 2 And 3 Levels B Calculate The Orbital Period In Each Of These Levels

The Bohr Model Of The Atom Ck 12 Foundation

Bohr S Model Of An Atom With Postulates And Limitations Byju S

How To Draw The Bohr Rutherford Diagram For Boron Youtube
2
Pdf Bohr Model Of The Hydrogen Atom
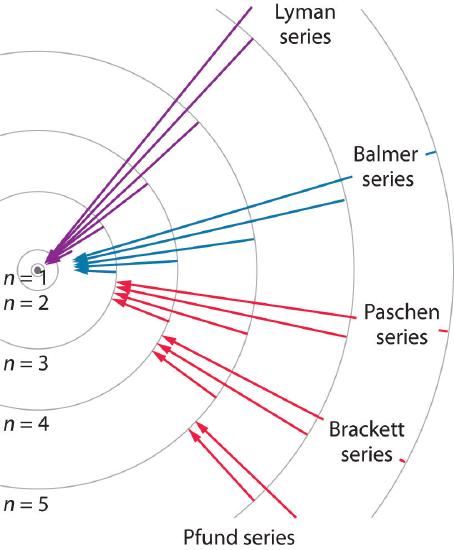
7 3 Atomic Spectroscopy And The Bohr Model Chemistry Libretexts
Q Tbn 3aand9gcsdmo7 Hb9nckvxvj3e2w2vpmegwcharh9h4tptjner9s75quof Usqp Cau
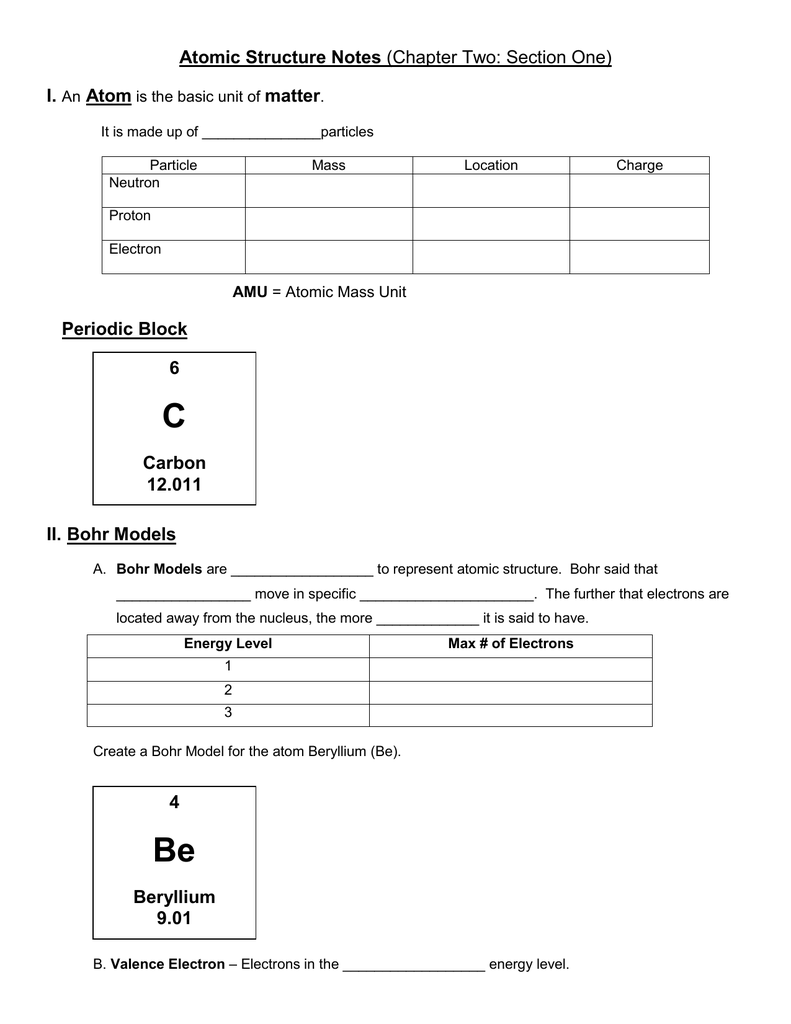
C Atomic Structure Notes I Atom

Bohr S Theory Of The Hydrogen Atom Physics
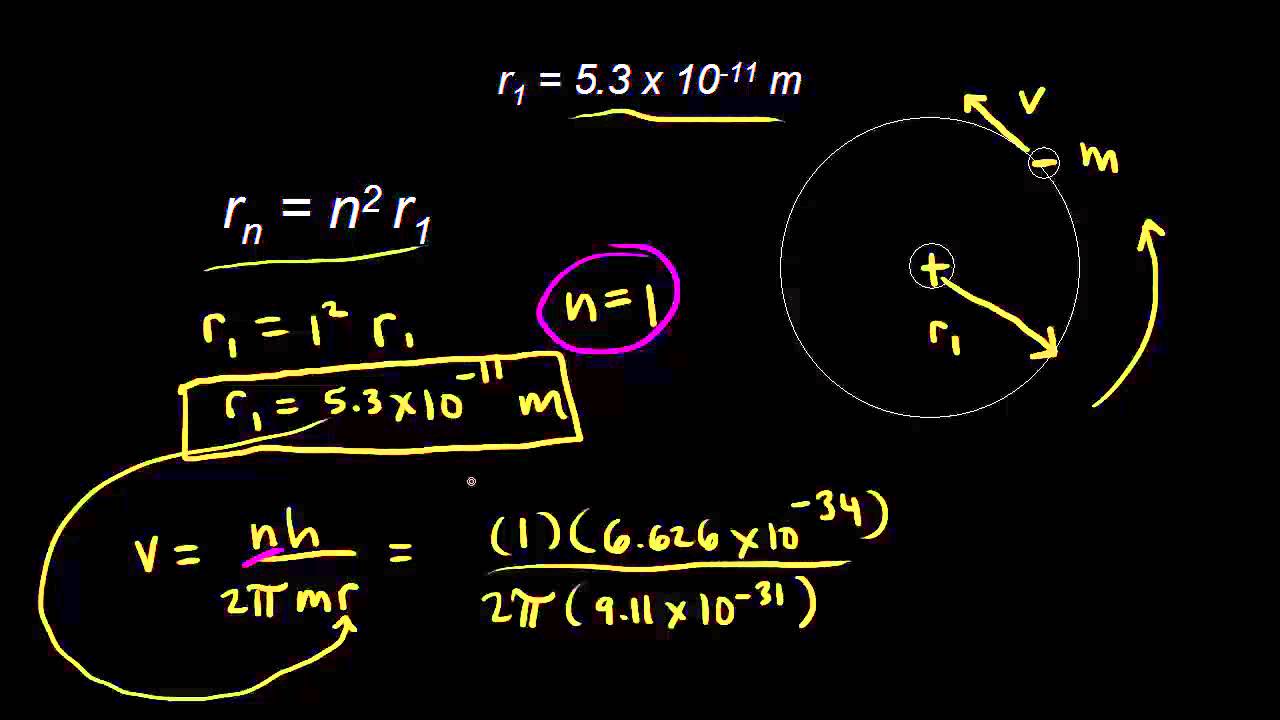
Bohr Model Radii Video Khan Academy
What Are The Main And Most Important Postulates Of Bohr S Atomic Model Quora
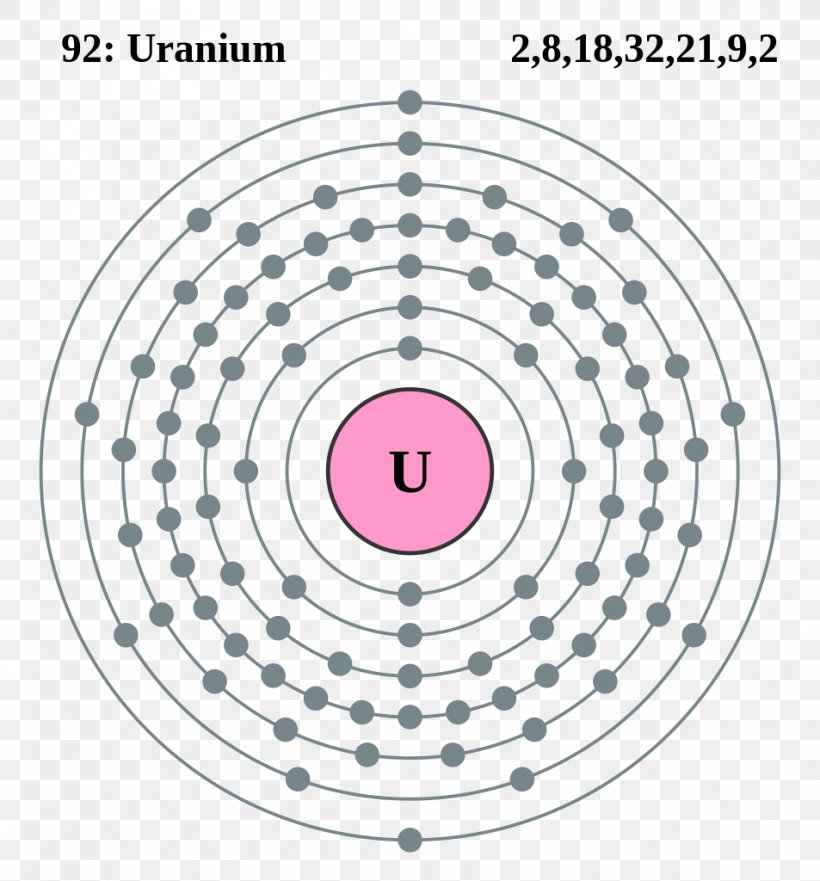
Electron Shell Uranium Bohr Model Electron Configuration Atom Png 953x1024px Electron Shell Actinide Area Atom Atomic

Sch 3ui Unit 2 Outline Up To Quiz 1 Atomic Theory And The Periodic Table Pdf Free Download

Ch 2 Test

Boron Sulfide American Elements
Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Atoms review sheet answer key Pdf
What Are The Main And Most Important Postulates Of Bohr S Atomic Model Quora

De Broglie And The Bohr Model
Bohr Diagrams Of Atoms And Ions Chemistry Libretexts
Http Www Newarkcatholic Org Wp Content Uploads 14 09 Bohr Model Ws Key1 Pdf

Hxfttwrs0mqbym

Bohr S Model Of An Atom With Postulates And Limitations Byju S

Bohr Model Practice Orbitals Displayed 3 Worksheets 4 Versions 24 Pages

Bohr Model Finite Well Review Of Modern Physics Quiz Docsity

Ashish Arora Atoms Electron Atomic Nucleus

Bohr Model Aca Grade 8 Science

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

Bohr S Model Of An Atom Ppt Video Online Download
Q Tbn 3aand9gctze5hhybrw Kx2kqb5vjzcondzjwniuleelmc627ztyywkvcuu Usqp Cau
Rutherford Bohr Model Of The Atom Springerlink

Bohr Atomic Model Postulates Distribution Of Electrons Videos Examples

Atoms Molecules Lab The Biology Primer

The Bohr Model Of The Atom Ck 12 Foundation
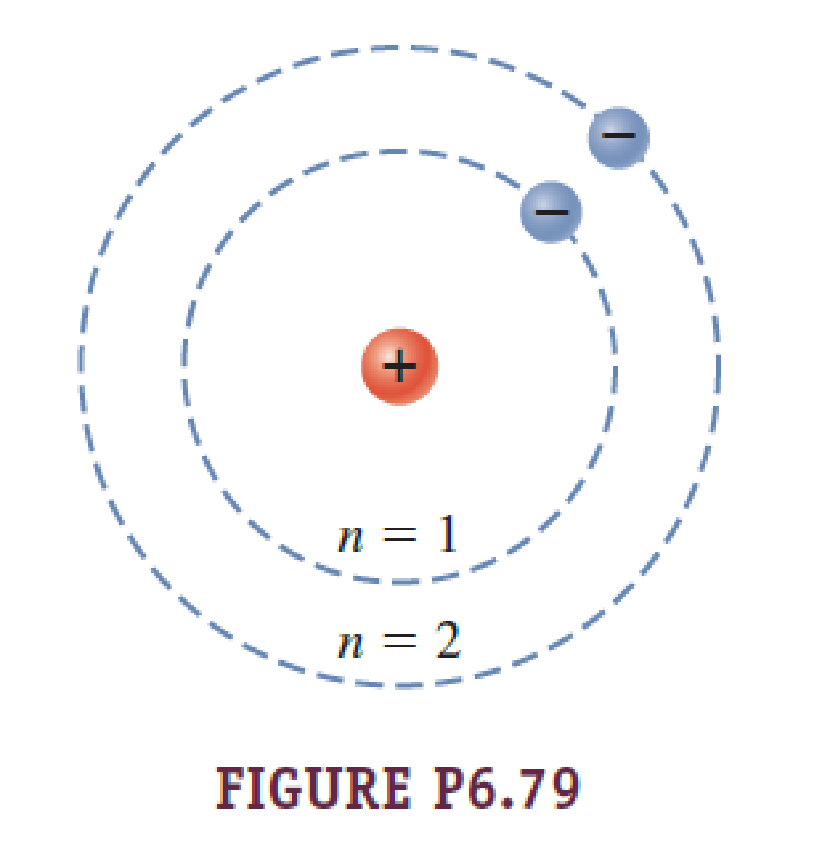
The Radius Of Circular Electron Orbits In The Bohr Model Of The Hydrogen Atom Are Given By 5 29 10 11 M N 2 Where N Is The Electron S Energy Level

The Bohr Model And Atomic Spectra Video Lesson Transcript Study Com
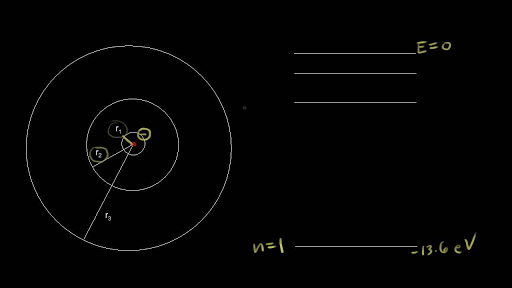
Bohr Model Energy Levels Video Khan Academy

Bohr Model Bohr Atomic Model Chemistry Tutorcircle Com Bohr Model Science Projects Atom Model Project

Bohr Model Description Development Britannica

10 Best Atomic Models Images Bohr Model Atomic Theory Atom Model

Solved In The Bohr Model Of The Helium Atom An Electron Chegg Com

Compare The 3 Atomic Models Proposed By Thomson Rutherford Amp Bohr Class 9 Brainly In
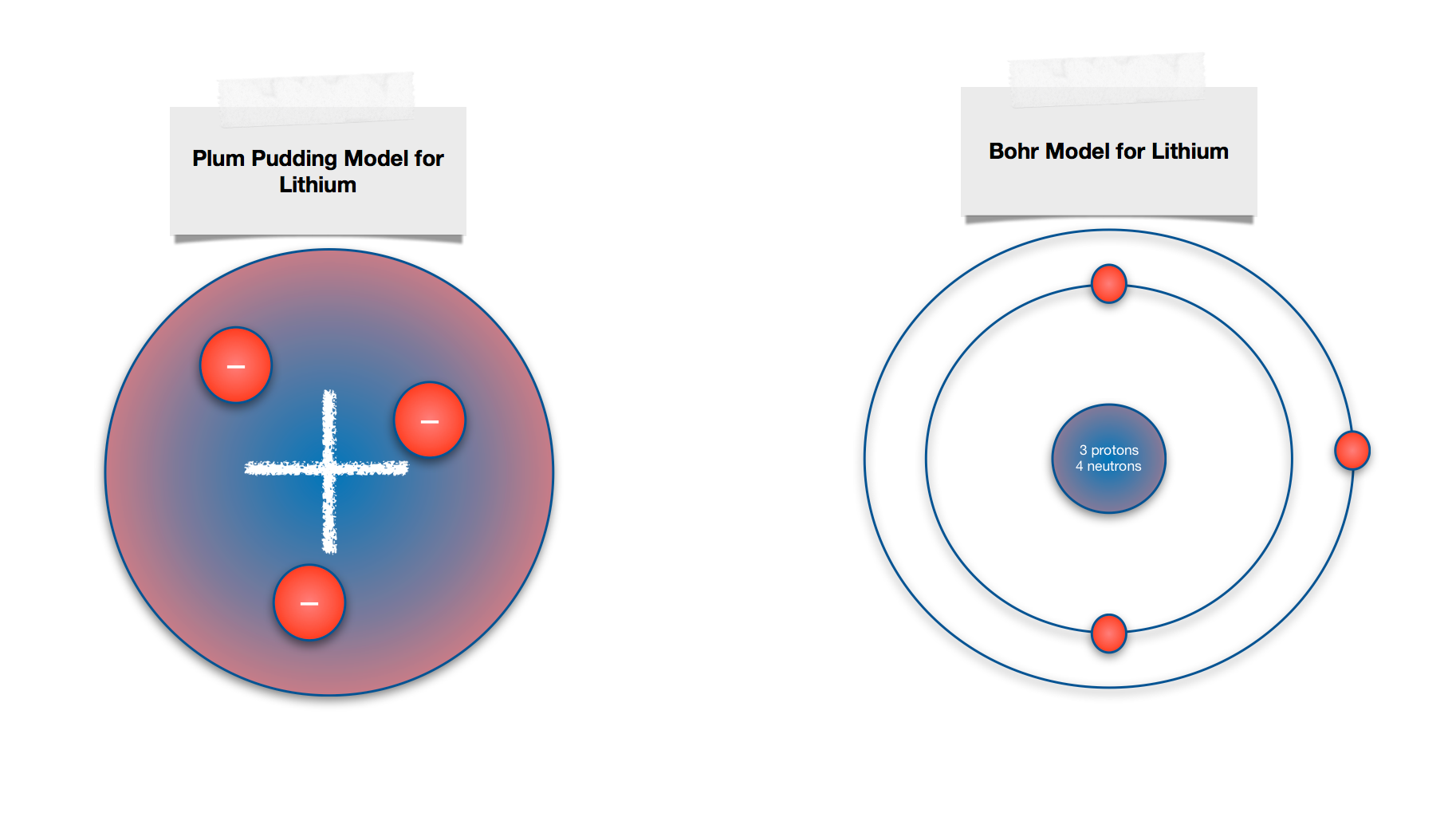
Explain The Similarities And Differences Between The Plum Pudding Model And The Bohr Model Of The Atom Draw A Sketch To Illustrate Your Answer Homework Help And Answers Slader
Misszukowski Weebly Com Uploads 9 7 4 6 4 Periodic Trends The Bohr Model Pdf

2 John Dalton Did His Research Work In Which Of The Following Countries A France B Greece C Russia D England Pdf Free Download
.png?width=430&name=atom-1674878_640%20(1).png)
Understanding The Bohr Atomic Model

The Bohr Model By Nathan Vittitoe
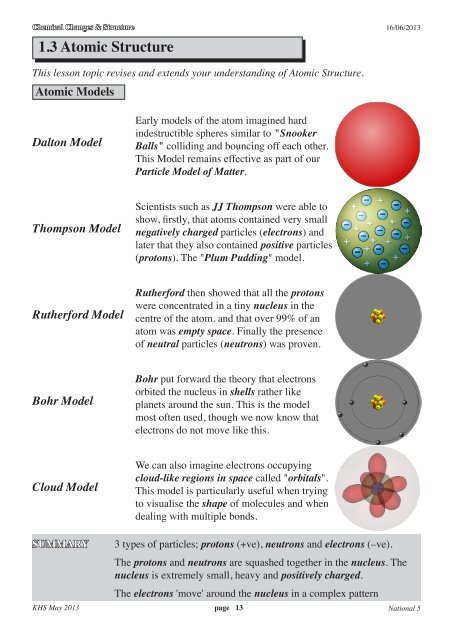
1 3 Atomic Structure Chemistry Teaching Resources

Solved Question 9 1 A Bohr Model Representation Of The H Chegg Com

Bohr Model Practice Vl 1 Ppt Download

Answer Which Energy Gap In The Bohr Model Clutch Prep

Amazon Com Niels Bohr Atom Model Hanging Mobile 9 Inches Steel Handmade In Denmark By Flensted Home Kitchen
Bohr Model Of The Atom Overview And Examples

Bohr S Theory Of The Hydrogen Atom Physics

How To Draw Bohr Models Youtube

The Energy Level Diagram Of The Hydrogen Atom Is Shown The Figure Below An Atom Generally Homeworklib
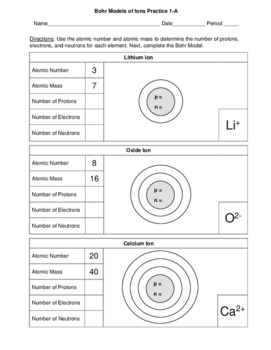
Bohr Models Of Ions 2 Worksheets 3 Skill Level Versions Of Each 12 Pages
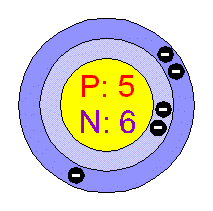
Chemical Elements Com Boron B
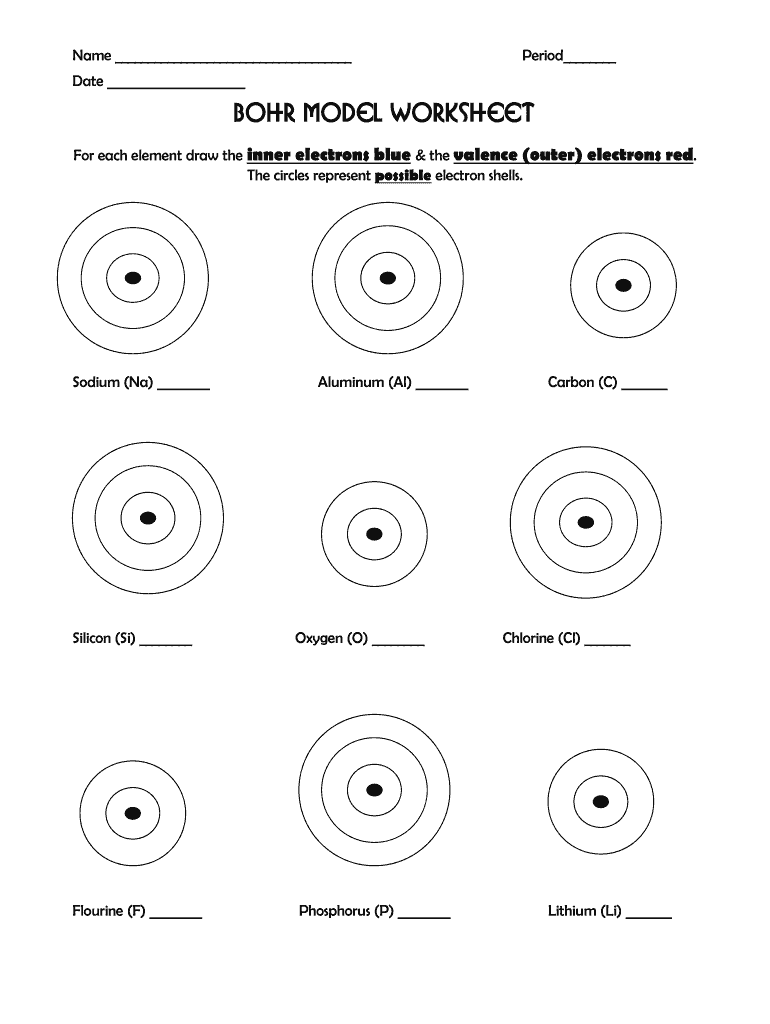
Bohr Model Worksheet Answers Fill Out And Sign Printable Pdf Template Signnow

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit
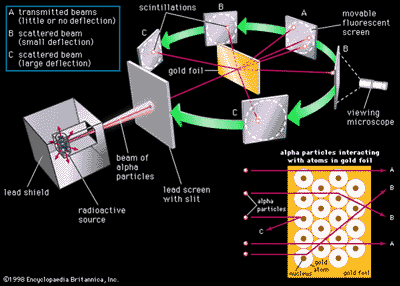
Ne 581 Radiation Protection Osu Extended Campus Oregon State University

Bohr Model And Ems Practice

Bohr Models Family 1 Interactive Worksheet

Bohr Model Practice With Cations Anions And Isotopes Bohr Model Atom Model Atom



